Matthew Smith
Postdoctoral Research Associate in the Burés group developing methods for the analysis of complex heterogeneous mixtures by NMR spectroscopy.Please use the above buttons for further information, or contact me at the links below.

About Me

My name is Matthew, i'm a postdoctoral research associate based at the University of Manchester, developing methods for the analysis of complex heterogeneous mixtures by NMR spectroscopy.My PhD involved studying the medicinal applications of Blatter-type radicals and related species. I was primarily based at Durham University undertaking synthesis of novel triazolium and Blatter-type radicals, but also undertook cellular assays within the Centre for Cancer at Newcastle University.Please see the 'My PhD Research' page for more information about my work.I grew up in Scarborough, England before undertaking a masters degree in medicinal chemistry at Newcastle University. As part of my masters I worked with Dr. Ian Hardcastle looking into covalent inhibition of a key tyrosine kinase for anticancer treatment. As the first in my close family to attend university I had an interest in promoting further study to those finishing their A levels, and as a result was a student ambassador for the 4 years of my undergraduate degree.When I'm not in a lab coat, i like to keep active; I particularly enjoy hiking and going to the gym. I also enjoy watching football, pub quizzes and the occasional bit of photography. Recently I've also been lucky enough to become a product reviewer with Amazon and find it very interesting looking at the diverse range of products they have available (it's also nice getting free stuff all the time!).I'd like to finish off with a fun fact about myself - when I was a school I won the award from my science teacher for 'most likely to become a scientist'.
My PhD Research

A photo including most of the lab members present during my PhD. The group undertake research generally within the fields of organocatalysis, peptide synthesis and mechanistic evaluation.Overview
My PhD project was titled 'Nitron Derivatives to Blatter Radicals: Novel Anticancer Prodrugs and Mechanistic Probes'. In short the project hoped to combine synthesis of Blatter-type radicals with biological evaluation to describe the potential applications of Blatter-type radicals as anticancer agents.There is also an additional interest in the organocatalytic properties of triazolium and cyclopropenium salts, the former a precursor in the commonly used synthesis of Blatter-type radicals (More information in the section 'synthesis of Blatter-type radicals).What are Blatter-type Radicals?
Benzo[e]-1,2,4-triazinyl radicals, or Blatter radicals, are a family of persistent radical species consisting of the bicyclic 1,2,4-benzotriazine skeleton. The first synthesis of a Blatter radical was detailed by Herbert Blatter and Halina Lewkazewski in a Tetrahedron Letters paper published in 1968, with a possible application as a calibration standard in electron paramagnetic resonance (EPR) proposed.Since then, research into Blatter-type radicals has highlighted a number of possible applications from semiconductors to molecular magnets and to treatments against a variety of disease classes. The variety of applications also opens opportunities of developing multifunctional materials, incorporating many of the uses of Blatter-type radicals. My interests were within the medicinal applications of Blatter-type radicals and this will be revisited below.Synthesis of Blatter-type Radicals
In 2017, the group published an article in Nature Communications detailing the facile transformation of nitron, within wet acetonitrile, to 3-amido-1,2,4-benzotriazinyl radicals. This route allows access to Blatter-type radicals difficult to obtain using conventional synthetic procedures.Link to Nature Communications article - New Blatter-type radicals from a bench stable carbeneBy synthesis of novel 1,2,4-triazolium salts described by the below general structure, it was hoped that we could undertake transformation of these triazoliums to Blatter-type radicals. As part of my work, varying the bridging heteroatom 'X' from N to S, we hoped to access 3-thioaryl-1,2,4-triazolium salts and 4-thiacinnolinyl radicals (S-centered Blatter-type radicals):

The formed 1,2,4-triazolium salts are also of interest for potential organocatalytic properties, given the potential formation of N-heterocyclic carbenes from C(3)-deprotonation.Applications of Blatter-type Radicals
As noted earlier, Blatter-type radicals have found a number of potential applications in fields of medicinal chemistry, magnetochemistry, photochemistry, polymer initiation and electrochemistry.My interests were primarily in the potential of Blatter-type radicals acting as anticancer agents, such applications were first highlighted in a 2018 Molecules paper. This paper shows micromolar potency of Blatter-type radicals against prostate (DU-145) and breast (MCF-7) cancer cell lines, with nanomolar potency achieved with the oxidized 1,2,4-benzotriazin-7-one analogues.Other work, such as that detailed in the 2012 Eur. J. Med. Chem. paper, has highlighted potential applications as BTRs acting as dual inhibitors of both acetyl/butyryl cholinesterases and the self-aggregation of beta-amyloid peptide. Such inihibiton can prevent the neurotoxicity and neurodegeneration resulting from Alzheimer's Disease.The involvement of Blatter-type radicals in the treatment of tuberculosis, malaria, leishmaniasis, Chagas Disease and cardiovascular disease has not been directly evaluated. However, multiple publications have implied such reactive species play a role in potential treatments for these conditions.Kinetic Evaluation of Triazolium and Cyclopropenium Salts
The field of chemistry is progressing away from toxic, expensive metal catalysts in synthesis, towards benign organocatalyst species that function within environmentally friendly solvents such as water and simple alcohols. The relevance of this work is clear given the recognition of asymmetric organocatalysis in awarding the Nobel Prize in Chemistry 2021 to David MacMillan and Benjamin List.N-heterocyclic carbenes are the most common organocatalyst family implemented in a range of synthetically important transformations such as the benzoin and Stetter reactions. Within this project the interest is in 1,2,4-triazolium (1) and cyclopropenium (2) salts which are the precursors to triazolyl (3) and cyclopropenyl (4) N-heterocyclic carbenes, obtained by deprotonation.

Through kinetic evaluation of the H/D exchange reactions of 1,2,4-triazolium and cyclopropenium salts, we could obtain valuable mechanistic insight of the links between catalyst design and efficiency. This allows for potential development of effective organocatalysts for use in pharmaceutical development and other areas of industrial synthesis.
Publications
Latest ArticleTriazolium Salt Organocatalysis: Mechanistic Evaluation of Unusual Ortho-Substituent Effects on Deprotonation
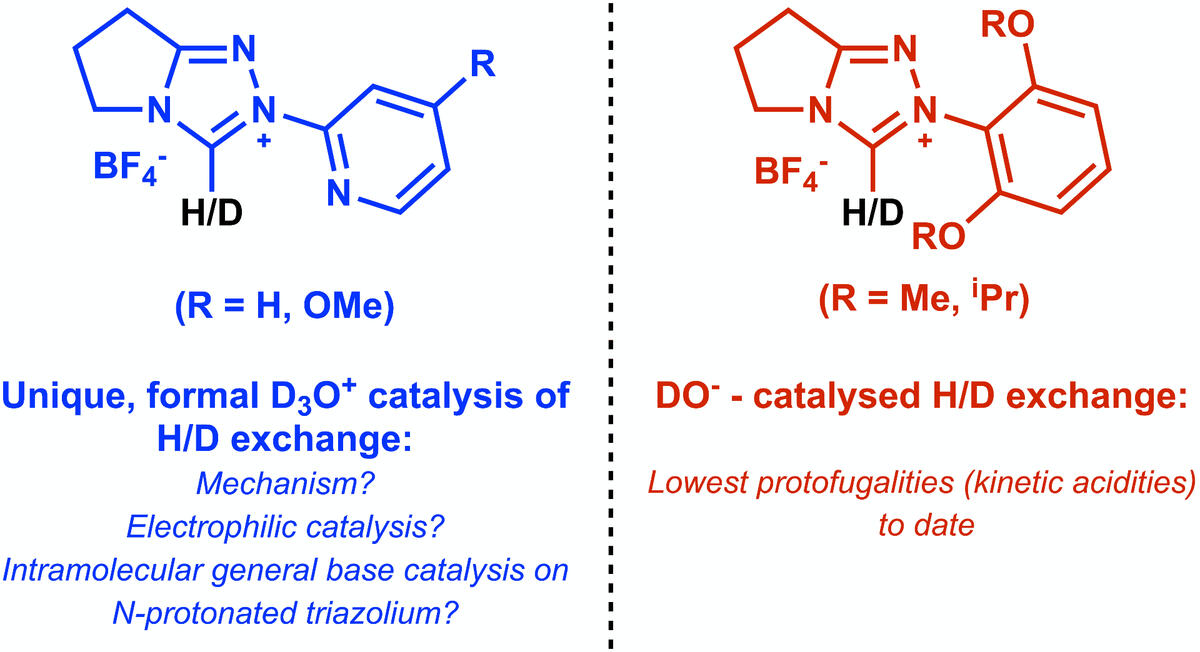
Organocatalysis by N-heterocyclic carbenes is normally initiated by the deprotonation of precursor azolium ions to form active nucleophilic species. Substituent effects on deprotonation have an impact on catalytic efficiency and provide insight into general catalytic mechanisms by commonly used azolium systems. Using an NMR kinetic method for the analysis of C(3)-H/D exchange, we determined log k(ex)–pD profiles for three ortho-substituted N-aryl triazolium salts, which enables a detailed analysis of ortho-substituent effects on deprotonation. This includes N-5-methoxypyrid-2-yl triazolium salt 7 and di-ortho-methoxy and di-ortho-isopropoxyphenyl triazolium salts 8 and 9, and we acquired additional kinetic data to supplement our previously published analysis of N-pyrid-2-yl triazolium salt 6. For 2-pyridyl triazoliums 6 and 7, novel acid catalysis of C(3)-H/D exchange is observed under acidic conditions. These kinetic data were supplemented by DFT analyses of the conformational preferences of 6 upon N-protonation. A C(3) deprotonation mechanism involving intramolecular general base deprotonation by the pyridyl nitrogen of the N(1)-deuterated dicationic triazolium salt is most consistent with the data. We also report k(DO) values (protofugalities) for deuteroxide-catalyzed exchange for 6–9. The protofugalities for 8 and 9 are the lowest values to date in the N-aryl triazolium series.Link to article
List of Publications2022
CCDC 2204367: Experimental Crystal Structure Determination
M. S. Smith, A. C. O'Donoghue and D. S Yufit, CSD Communication, 2022
Link to articleCCDC 2204366: Experimental Crystal Structure Determination
M. S. Smith, A. C. O'Donoghue and D. S Yufit, CSD Communication, 2022
Link to article2021
Triazolium Salt Organocatalysis: Mechanistic Evaluation of Unusual Ortho-Substituent Effects on Deprotonation
P. Quinn, M. S. Smith, J. Zhu, D. R. W. Hodgson and A. C. O'Donoghue, Catalysts, 2021, 11, 1055
Link to article
Contact
Please use the links below to contact me. I'm happy to discuss my research, potential collaboration opportunities, industrial placements or just have a general chat.